support@comsol.com
Chemical Reaction Engineering Module Updates
For users of the Chemical Reaction Engineering Module, COMSOL Multiphysics® version 6.2 brings enhancements to the modeling of gas–liquid equilibria involving concentrated mixtures with two or more components, as well as new functionality for consistent initialization and new capabilities for parameter estimation. Read more about these updates below.Equilibrium for Vapor–Liquid Interfaces
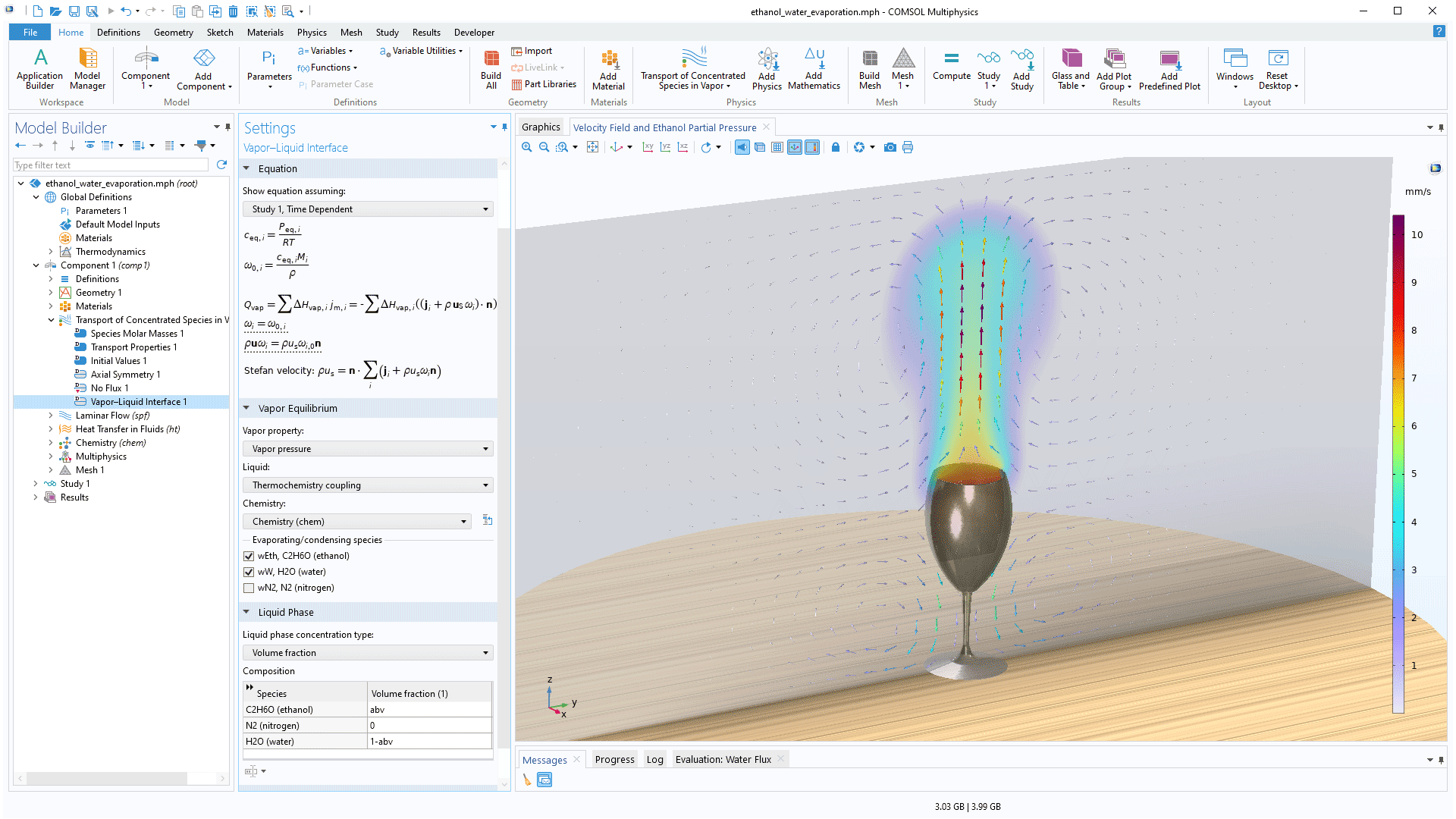
New capabilities have been added for modeling gas–liquid equilibria involving concentrated solutions. This functionality is available as three new extensions to the Transport of Concentrated Species interface: Vapor Inflow (for modeling laminar flow and vapor equilibria at pure liquid surfaces), Vapor–Liquid Interface (for modeling upstream inlets and laminar flow with moving mesh), and Vapor–Liquid-Mixture Interface (for modeling turbulent flow and vapor–liquid mixture phase boundaries). Additionally, a new set of multiphysics interfaces for vaporization and condensation at a moving vapor–liquid interface is available for modeling two-phase flow with moving mesh. These additions make it easier to model vapor–liquid equilibria and allow for including condensation and evaporation in multiphase flow models, which previously had to be done manually. You can see these additions in use in the Evaporation of Ethanol and Water from a Wine Glass tutorial model.
Consistent Initialization of Chemical Equilibrium
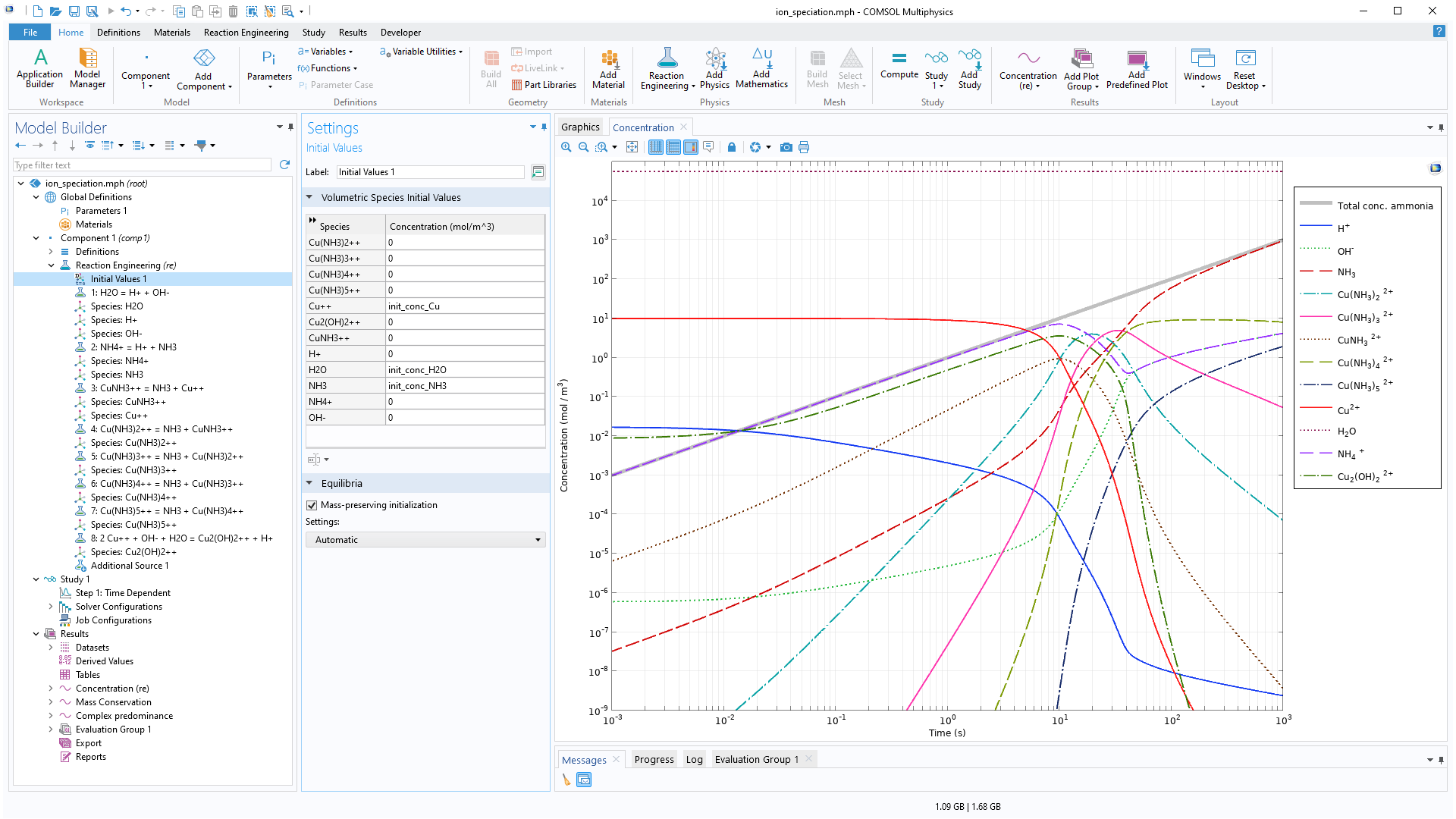
For equilibrium reactions, a new Mass-preserving initialization feature can be enabled in the Initial Values node of the Reaction Engineering interface, substantially increasing accuracy and robustness. Enabling this feature makes it easier to define chemical equilibria without the need to manually compute an initial state. This is especially useful for complex reaction mechanisms where several of the reactions are assumed to be at equilibrium. View this addition in the new Acid–Base Equilibria and Copper Speciation in Ammonia Solution tutorial model.
Access to Parameter Estimation Functionality
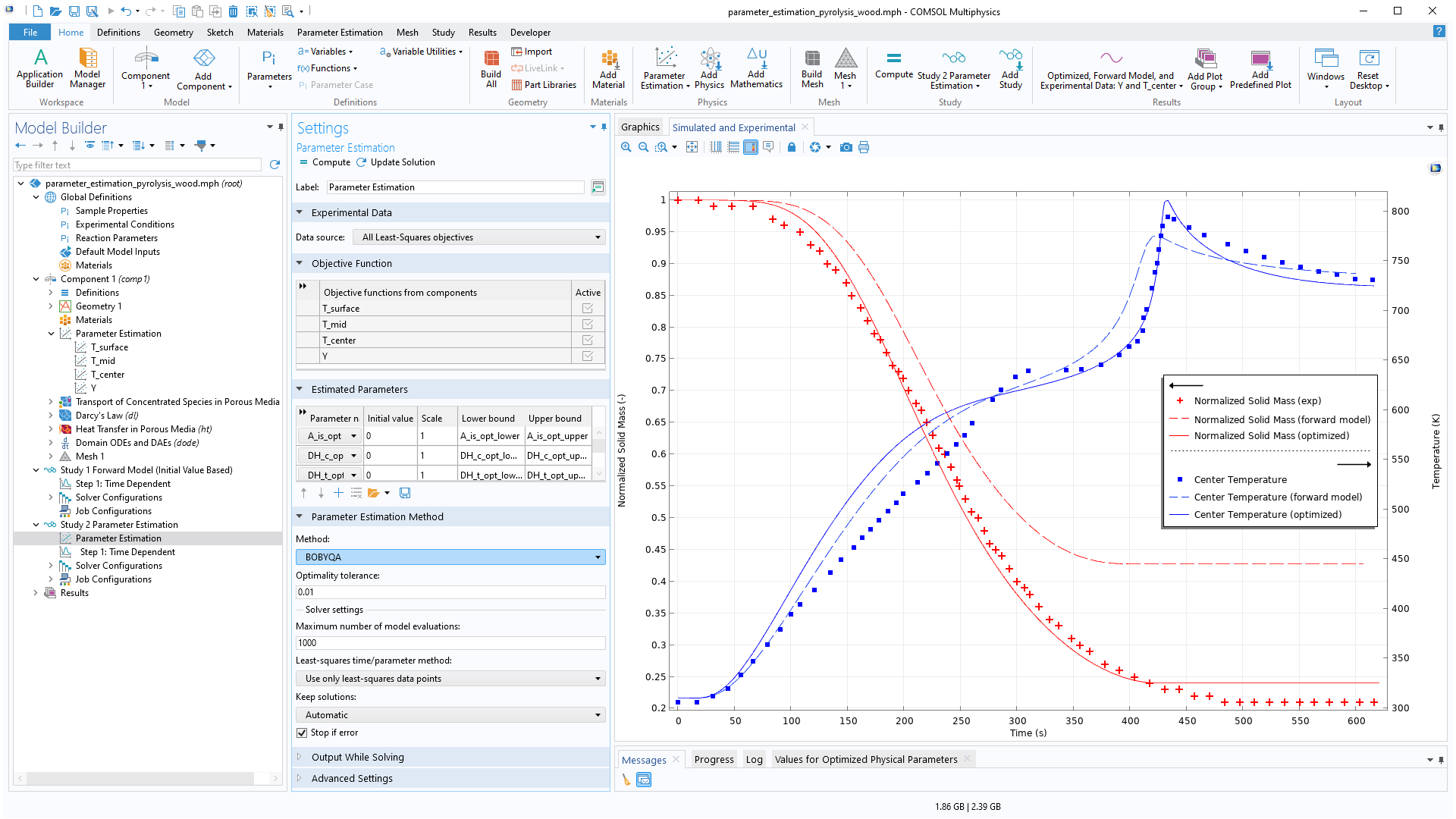
The Chemical Reaction Engineering Module has been expanded with new capabilities for parameter estimation that previously required the Optimization Module. Functionality for defining multiple objectives from experimental data is now included, as well as a range of solvers, including the gradient-based IPOPT and Levenberg–Marquardt solvers and the gradient-free BOBYQA solver. You can see this functionality in use within the new Pyrolysis of Wood tutorial model.
Nonisothermal Reacting Flow in Porous Media
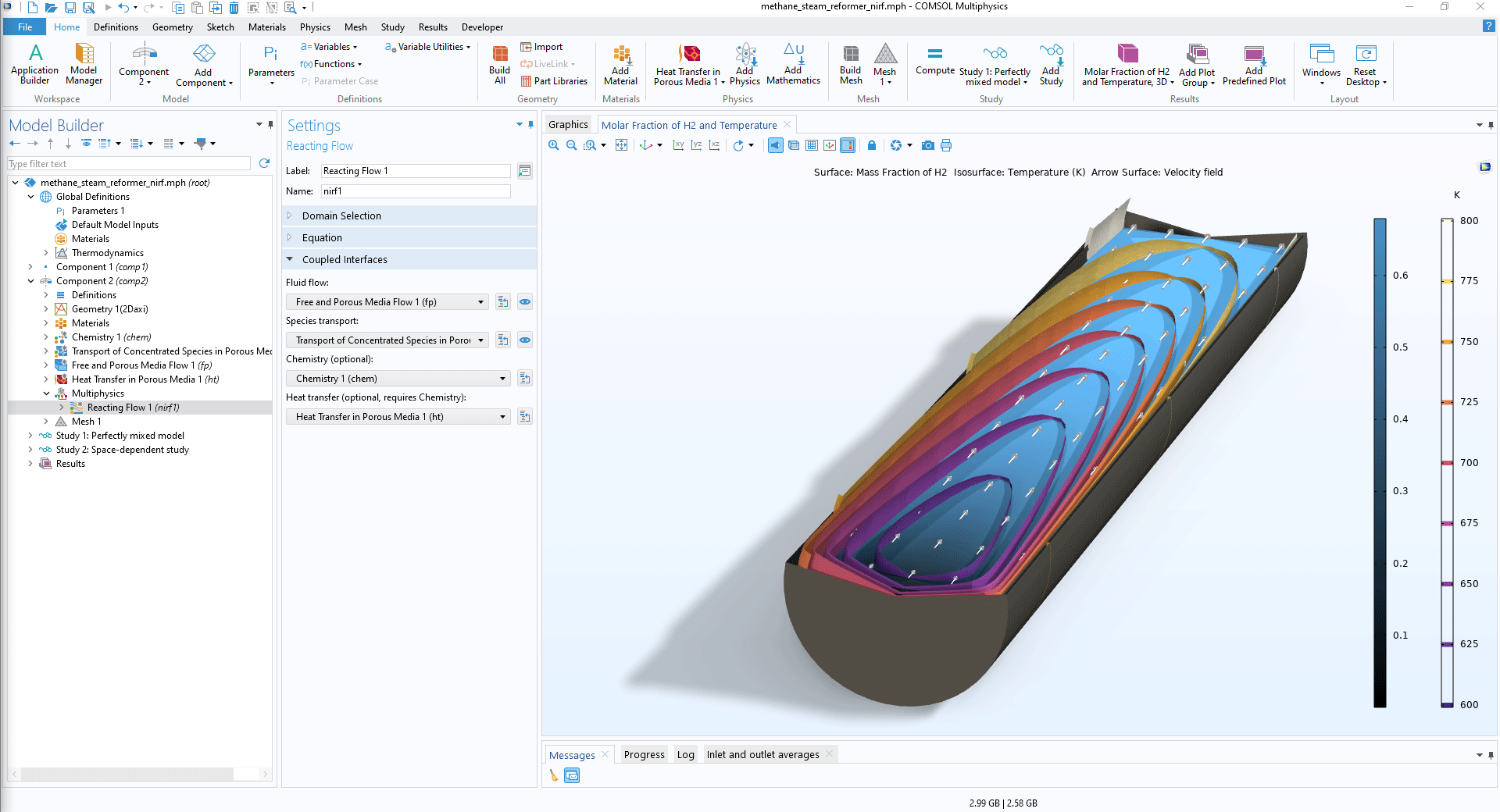
You can now model nonisothermal reacting flow in porous media with the Reacting Flow coupling feature. Because this feature now fully supports porous domains, you are no longer required to include both a Reacting Flow and a Nonisothermal Flow coupling feature, and you can also now use this feature in combination with the Heat Transfer in Porous Media interface. With the new approach available in version 6.2, you get consistent mass and heat transfer due to the fact that all connected physics (fluid, mass, and heat) use the same thermodynamics, defined in a Chemistry interface. In addition, heat conservation is now more accurate since enthalpy diffusion is automatically included. Note that to use this functionality, you need to add a Porous Medium feature to each of the three transport physics interfaces: Transport of Concentrated Species, Brinkman Equations, and Heat Transfer in Porous Media.
In the Transport of Concentrated Species interface, the Porous Medium and Porous Catalyst features are now supported. Reactions in a fluid are facilitated by both features, and reactions on the surface of a porous matrix are enabled by the latter. In the interfaces for heat transfer, the Local thermal equilibrium and Local thermal nonequilibrium options are now supported.
The new Nonisothermal Reacting Flow in a Methane Steam Reformer tutorial model demonstrates the new functionality of the Reacting Flow coupling.
New Tutorial Models
COMSOL Multiphysics® version 6.2 brings several new tutorial models to the Chemical Reaction Engineering Module.
Parameter Estimation for Pyrolysis of Wood
Application Library Title:
parameter_estimation_pyrolysis_wood
Download from the Application Gallery
Precipitation of Barium Sulfate
Application Library Title:
BaSO4_crystallization
Download from the Application Gallery
Thermal Decomposition of Beta-Carotene in a Flow Reactor*
Application Library Title:
thermal_decomposition_uq
Download from the Application Gallery
*Requires the Uncertainty Quantification Module
Acid–Base Equilibria and Copper Speciation in Ammonia Solution
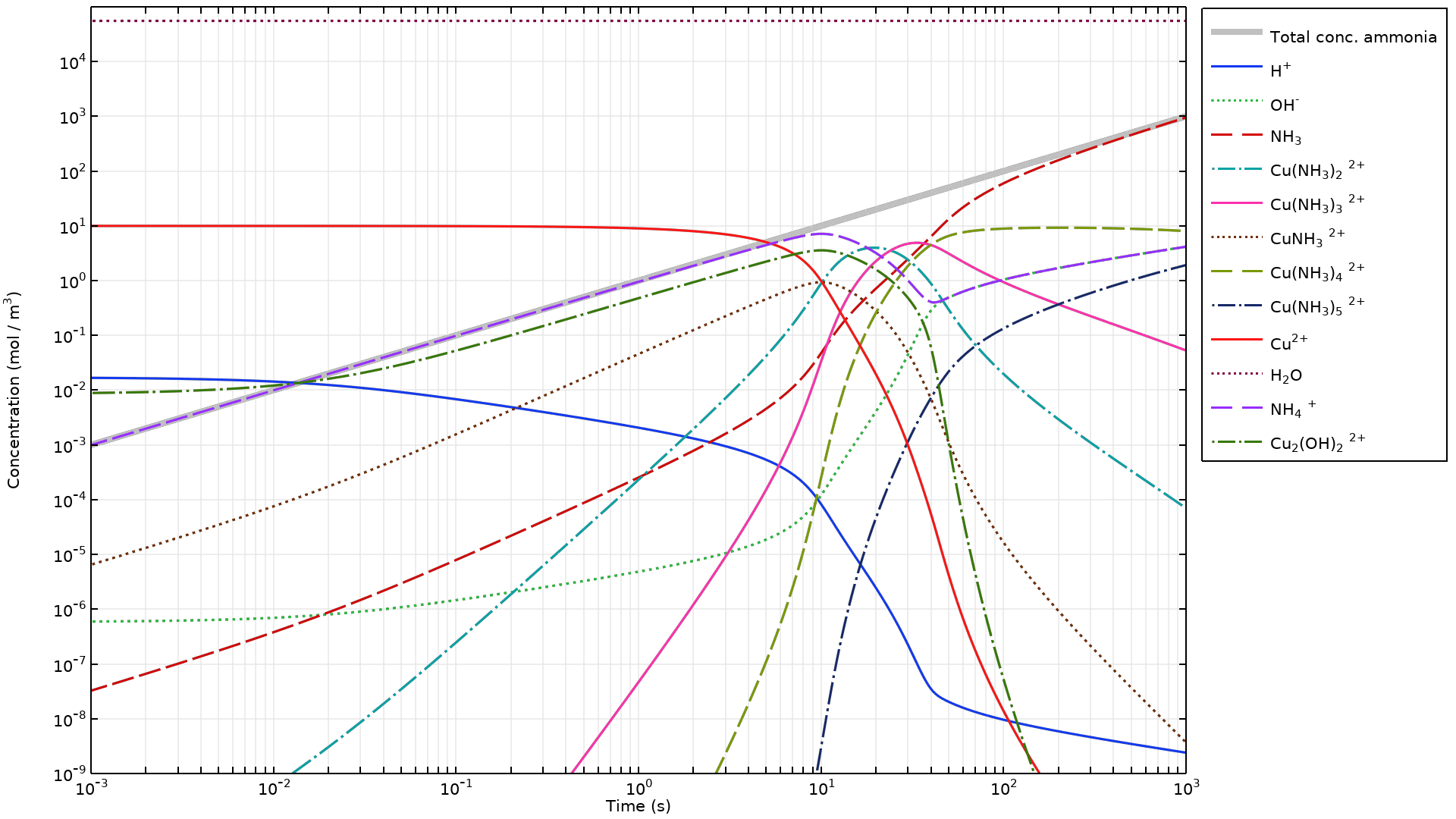
Application Library Title:
ion_speciation
Download from the Application Gallery
Nonisothermal Reacting Flow in a Methane Steam Reformer